For the biomechanics experts at Cetim, per-forming tests on hip or shoulder implants is part of their usual activity. But validating the mechanical performance of an implant made of silicone and textile turns out to be a more complex activity. And this is exactly what Cousin Biotech asked for, with the test of its first device intended to be used for surgical treatment of male urinary incontinence. “It is absolutely essential to perform fatigue tests in order to prove that our implant is safe. This is why we resorted to Cetim, as they have the required experience and the necessary testing equipment”, explains Gilles Solecki, project mana-ger at the Northern France company.
A specific test bench
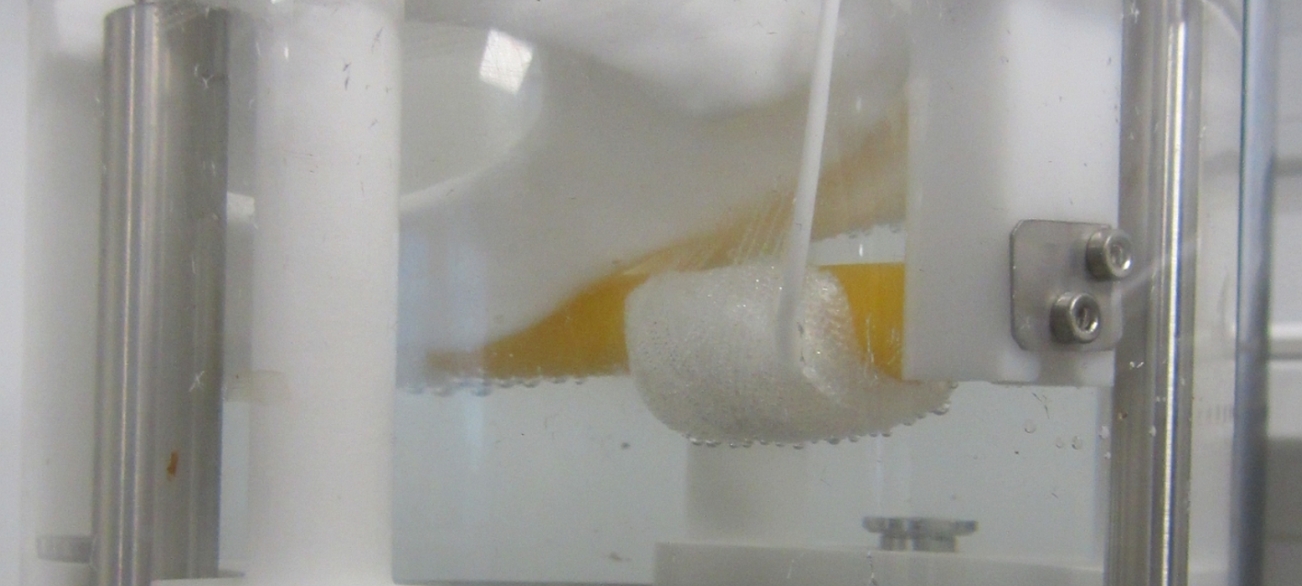
Basically, this innovative implant consists of a cuff, surrounded with technical textile, whose function is to continuously apply an adjustable pressure on the urethra and simultaneously limit its volume expansion. The pressure applied by the cuff on the urethra stops the incontinence. In order to test the implant, Cetim defined the representative stressing protocol and testing en-vironment in collaboration with Cousin Biotech and designed a specific test bench which reproduces the pelvis where the implant is placed and the implant’s sequence of operation, in a saline medium at a temperature of 37°C. The pelvis was made by additive manufacturing, while the soft tissues which interact with the implant were made of polymer materials. One of the major difficulties was related to the low level of physiological forces involved. A few tens of thousands of gradually increasing loading cycles were applied in order to simulate the operation of the implant over a period of 15 years. The measurement of the forces and displacements (approximately 5 mm) of the soft tissues, together with visual inspections and leak checks with a dye allowed Cetim to validate the mecha-nical performance of the implant. “The tests performed on two implants were perfectly conclusive” added Gilles Solecki. The report supplied by Cetim was then used to support the technical record of that medical device, which was implanted for the first time into a patient in July 2018.